Plot SNP array raw data
In addition to genotyping, SNP arrays are good tools for copy number calling. To do that, we need to take into account not the individual genotypes of each SNP in the array, but the general shape and values of the raw data. Good tools exist for that, but visualizing the raw data is an important step and a quality control.
In this example we’ll use data from a cancer cell line obtained with an Illumina OmniExpress array. The data file has been downsampled to only 100k snps, but other from that is the standard “FinalReport” one gets from GenomeStudio.
library(karyoploteR)
dd <- read.table(file = "Examples/data/SNPArray.26T.txt.gz", sep="\t", header=TRUE, stringsAsFactors = FALSE)
head(dd)
## SNP.Name Sample.ID Allele1...Top Allele2...Top GC.Score Sample.Name
## 1 rs11152336 34 A C 0.7622 26T
## 2 rs4458740 34 A C 0.8454 26T
## 3 rs4264565 34 C C 0.8317 26T
## 4 rs4474684 34 G G 0.7729 26T
## 5 rs7677662 34 A A 0.7739 26T
## 6 rs4682434 34 A C 0.4395 26T
## Sample.Group Sample.Index SNP.Index SNP.Aux Allele1...Forward
## 1 26T 32 81073 0 A
## 2 26T 32 443708 0 A
## 3 26T 32 434421 0 C
## 4 26T 32 444477 0 C
## 5 26T 32 613842 0 A
## 6 26T 32 456548 0 T
## Allele2...Forward Allele1...Design Allele2...Design Allele1...AB
## 1 C A C A
## 2 C T G A
## 3 C G G B
## 4 C C C B
## 5 A A A A
## 6 G A C A
## Allele2...AB Allele1...Plus Allele2...Plus Chr Position GT.Score
## 1 B A C 18 59864223 0.7679
## 2 B A C 7 156089537 0.8203
## 3 B C C 2 215283259 0.8107
## 4 B C C 16 951045 0.7734
## 5 A A A 4 145513053 0.7741
## 6 B T G 3 112534069 0.7903
## Cluster.Sep SNP ILMN.Strand Customer.Strand Top.Genomic.Sequence
## 1 0.7687 [A/C] TOP TOP NA
## 2 0.7304 [T/G] BOT TOP NA
## 3 1.0000 [T/G] BOT TOP NA
## 4 0.9690 [T/C] BOT BOT NA
## 5 0.9954 [A/G] TOP TOP NA
## 6 0.6738 [A/C] TOP BOT NA
## Plus.Minus.Strand Theta R X Y X.Raw Y.Raw B.Allele.Freq
## 1 + 0.651 0.642 0.243 0.398 1266 1753 0.6157
## 2 - 0.615 1.137 0.465 0.672 2350 2903 0.4573
## 3 - 0.972 1.266 0.053 1.213 486 5697 1.0000
## 4 + 0.974 1.161 0.046 1.115 432 4710 1.0000
## 5 + 0.033 1.369 1.300 0.068 6213 460 0.0000
## 6 - 0.330 0.465 0.296 0.169 1744 866 0.3379
## Log.R.Ratio CNV.Value CNV.Confidence GC_probe GCC_LRR
## 1 -0.3307 NA NA 0.31 -0.26
## 2 0.1754 NA NA 0.44 0.18
## 3 -0.0784 NA NA 0.31 -0.01
## 4 -0.1139 NA NA 0.62 -0.15
## 5 -0.0547 NA NA 0.36 -0.01
## 6 -0.1655 NA NA 0.30 -0.10
to plot it we’ll first extract the information we need and convert it into a GRanges using the toGRanges function from regioneR and setting start and end to the snp position. The data we need is the B-allele frequency (BAF), that is, the portion of the signal coming from the allele labeled as B, and the Log R Ratio (LRR), that is, the total amount of signal. We’ll need to change the chromosome names from the Ensembl style to the UCSC style, since we are using the standard hg19 genome.
snp.data <- toGRanges(dd[,c("Chr", "Position", "Position", "B.Allele.Freq", "Log.R.Ratio")])
names(mcols(snp.data)) <- c("BAF", "LRR")
seqlevelsStyle(snp.data) <- "UCSC"
head(snp.data)
## GRanges object with 6 ranges and 2 metadata columns:
## seqnames ranges strand | BAF LRR
## <Rle> <IRanges> <Rle> | <numeric> <numeric>
## [1] chr18 59864223 * | 0.6157 -0.3307
## [2] chr7 156089537 * | 0.4573 0.1754
## [3] chr2 215283259 * | 1 -0.0784
## [4] chr16 951045 * | 1 -0.1139
## [5] chr4 145513053 * | 0 -0.0547
## [6] chr3 112534069 * | 0.3379 -0.1655
## -------
## seqinfo: 26 sequences from an unspecified genome; no seqlengths
And we are ready to plot. We’ll create a scatter plot using the kpPoints function and we’ll plot the BAF in the upper part of the data panel (r0=0.5 and r1=1) and the LRR in the lower part (r0=0 and r1=0.5).
kp <- plotKaryotype()
kpPoints(kp, data=snp.data, y=snp.data$BAF, r0=0.5, r1=1)
kpPoints(kp, data=snp.data, y=snp.data$LRR, r0=0, r1=0.5)
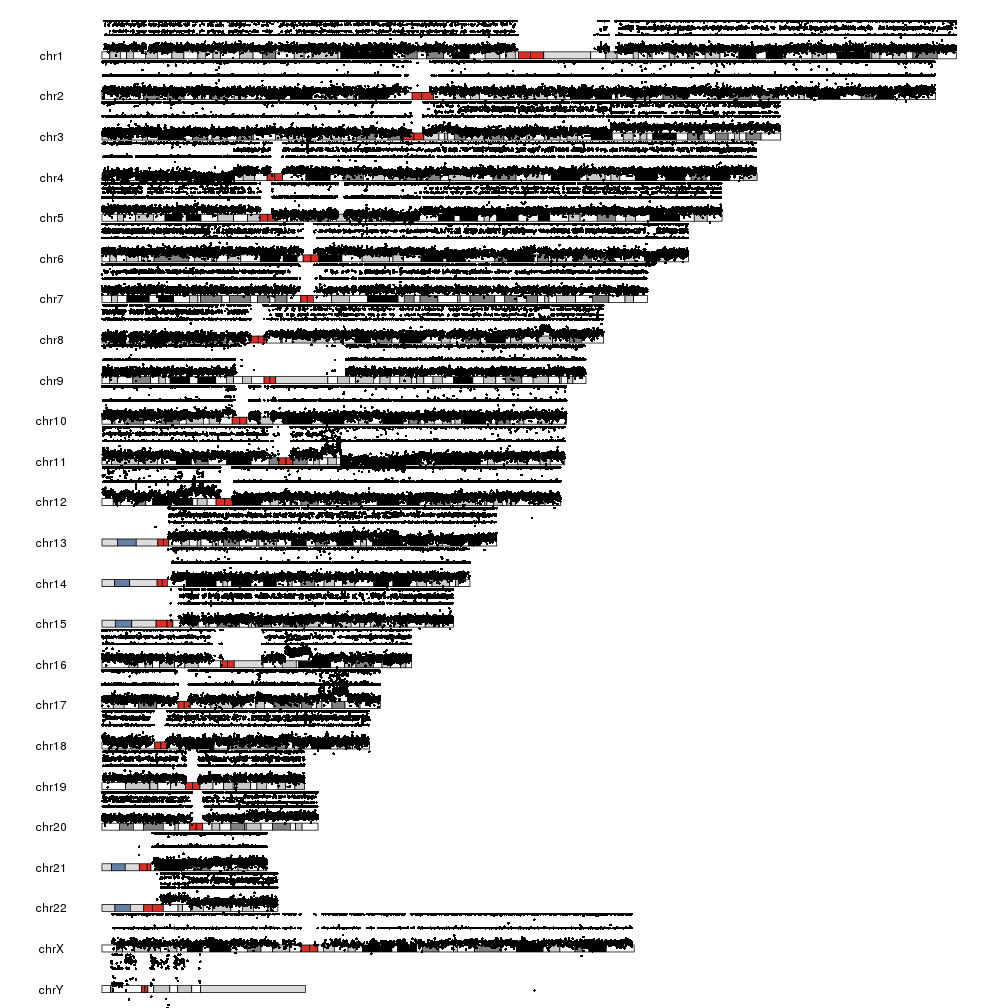
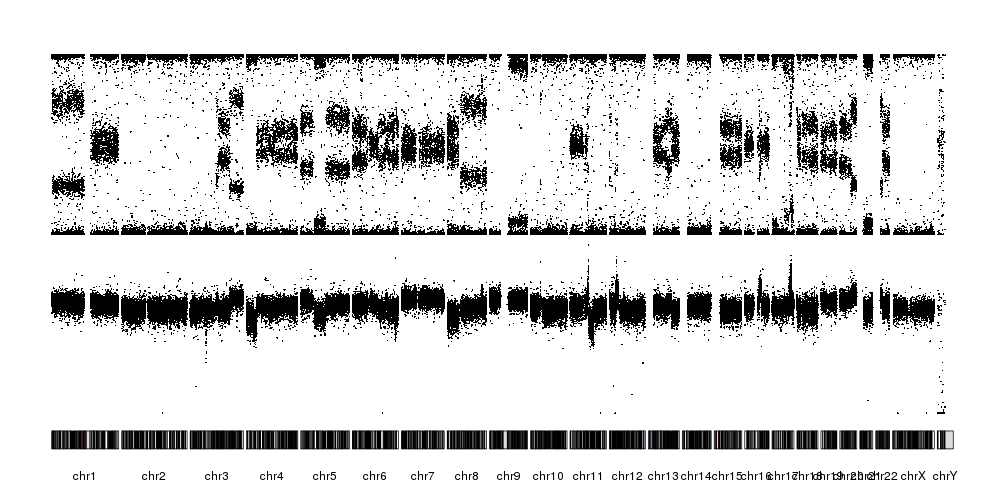
There are a few problems with this plot. First, we have BAF is in the [0,1] range but LRR is not, and it’s bleeding over the ideograms. Second, the points are too big to reveal the patterns, specially with the chromosomes one over the other. To solve this, we’ll change to another plot type with all chromosomes in a line, plot.type=4. We’ll deal with the overflowing LRR by setting a different ymin and cutting the points below that.
lrr.below.min <- which(snp.data$LRR < -3)
snp.data$LRR[lrr.below.min] <- -3
kp <- plotKaryotype(plot.type = 4)
kpPoints(kp, data=snp.data, y=snp.data$BAF, cex=0.3, r0=0.5, r1=1)
kpPoints(kp, data=snp.data, y=snp.data$LRR, cex=0.3, r0=0, r1=0.5, ymax=2, ymin=-3)
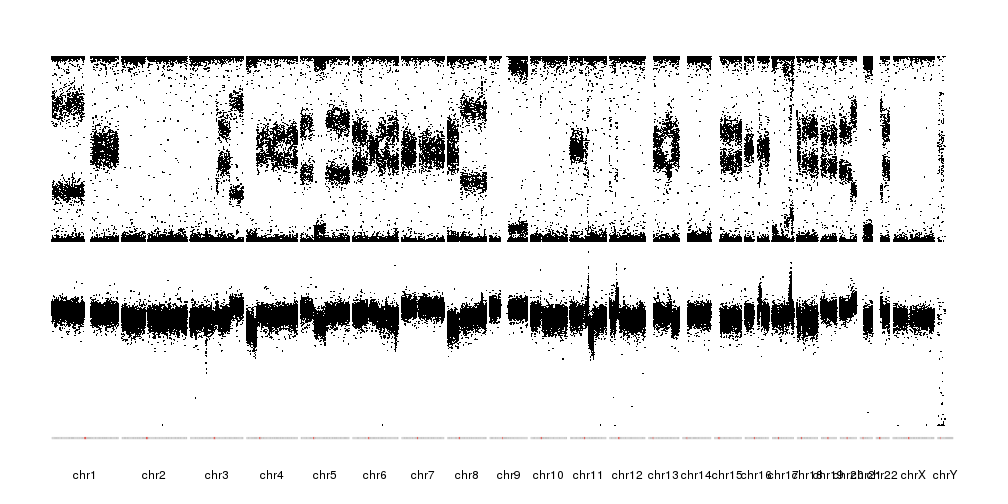
It is now possible to see the patterns of alteration, both in the BAF and the LRR. We can further refine the plot by reducing the margin between the data and the ideograms and changing the ideograms with a single line with the centromere.
pp <- getDefaultPlotParams(plot.type = 4)
pp$data1inmargin <- 2
kp <- plotKaryotype(plot.type = 4, ideogram.plotter = NULL, plot.params = pp)
kpAddCytobandsAsLine(kp)
kpPoints(kp, data=snp.data, y=snp.data$BAF, cex=0.3, r0=0.5, r1=1)
kpPoints(kp, data=snp.data, y=snp.data$LRR, cex=0.3, r0=0, r1=0.5, ymax=2, ymin=-3)
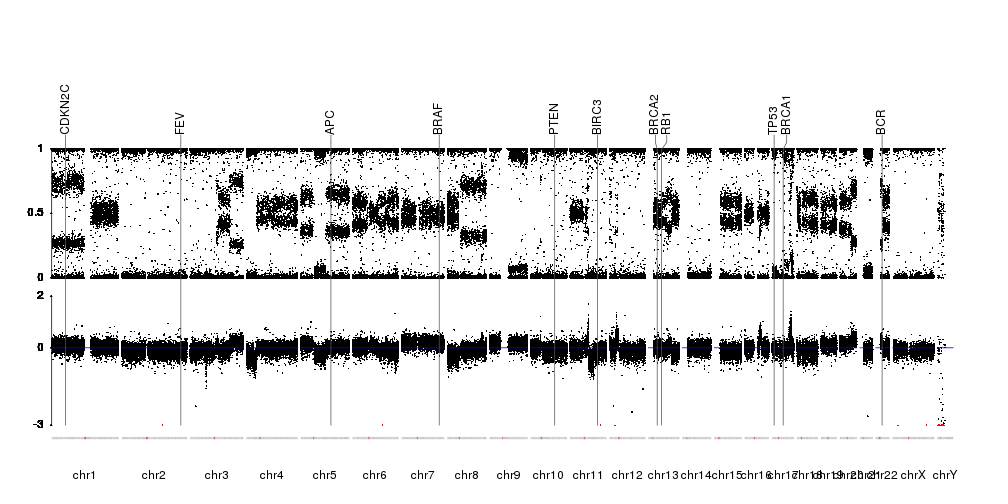
Finally, we’ll mark in red the points below ymin to mark they were cropped, we’ll add markers for a couple of genes, add axis were needed and a line marking the 0 LRR. The information on the position of the genes will come from Ensembl Biomart as in the Plot Genes example.
library(biomaRt)
gene.symbols <- c("AKT", "APC", "BCR", "BIRC3", "BRAF", "BRCA1", "BRCA2", "CDKN2C", "FEV", "TP53", "PTEN", "RB1")
ensembl <- useEnsembl(biomart="ensembl", dataset="hsapiens_gene_ensembl", version=67)
genes <- toGRanges(getBM(attributes=c('chromosome_name', 'start_position', 'end_position', 'hgnc_symbol'),
filters = 'hgnc_symbol', values =gene.symbols, mart = ensembl))
seqlevelsStyle(genes) <- "UCSC"
pp <- getDefaultPlotParams(plot.type = 4)
pp$data1inmargin <- 2
kp <- plotKaryotype(plot.type = 4, ideogram.plotter = NULL, plot.params = pp)
kpAddCytobandsAsLine(kp)
kpAxis(kp, r0=0.4, r1=0.75)
kpPoints(kp, data=snp.data, y=snp.data$BAF, cex=0.3, r0=0.4, r1=0.75)
kpAxis(kp, tick.pos = c(-3, 0, 2), r0=0, r1=0.35, ymax=2, ymin=-3)
kpPoints(kp, data=snp.data, y=snp.data$LRR, cex=0.3, r0=0, r1=0.35, ymax=2, ymin=-3)
kpPoints(kp, data=snp.data[lrr.below.min], y=snp.data[lrr.below.min]$LRR, cex=0.3, r0=0, r1=0.35, ymax=2, ymin=-3, col="red")
kpAbline(kp, h=0, r0=0, r1=0.35, ymax=2, ymin=-3, col="blue")
kpPlotMarkers(kp, data=genes, labels=genes$hgnc_symbol, line.color = "#555555", marker.parts = c(0.95,0.025,0.025), r1=1.05)